Foreign
COVID: US FDA approves Pfizer/BioNTech Covid-19 vaccine
Published
3 years agoon
By
Vida Essel-Lamptey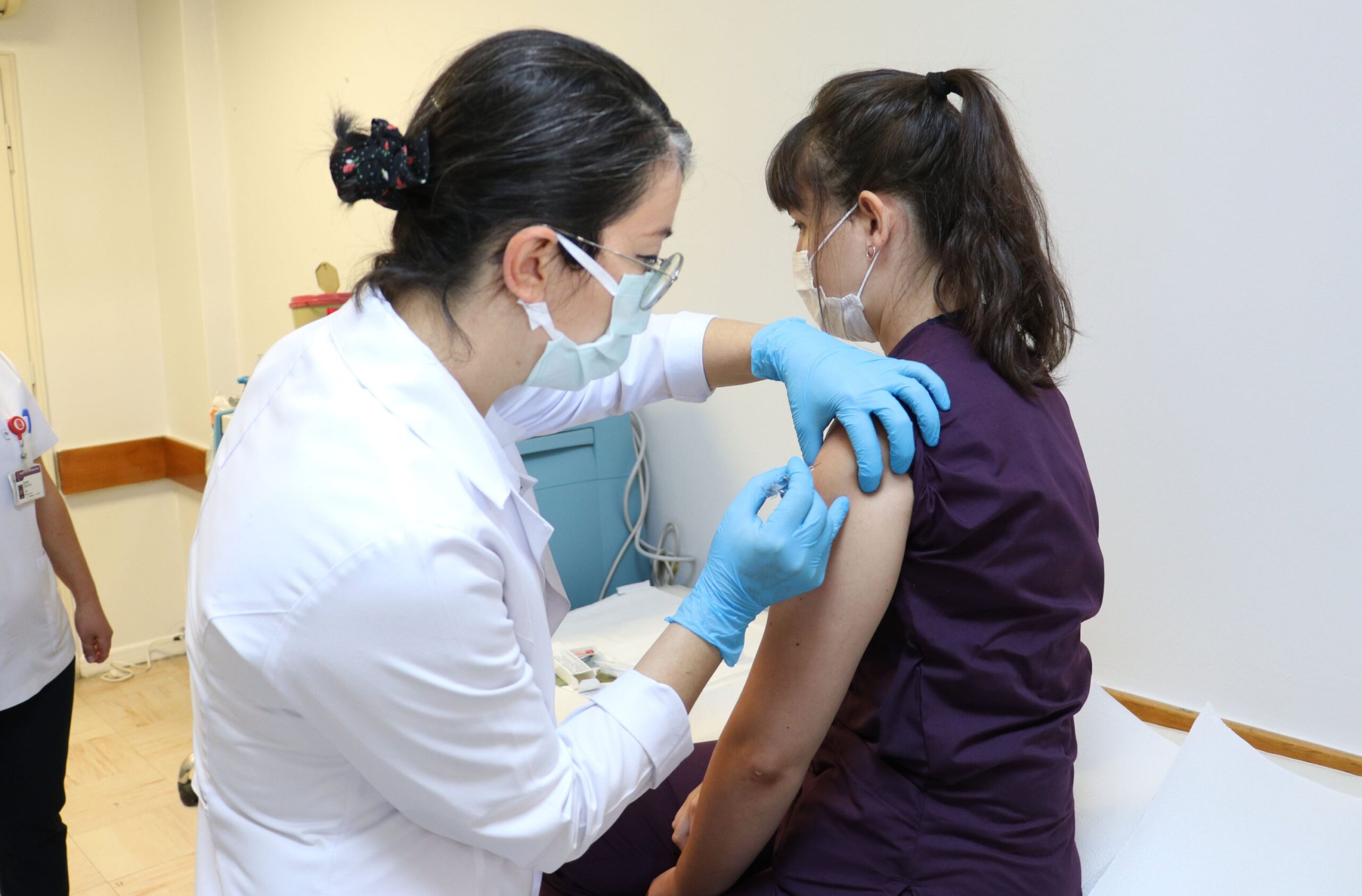
The United State of America’s Food and Drug Administration(FDA) has authorized the Pfizer and BioNTech coronavirus vaccine for an emergency use by the country in its fight against the deadly Covid-19 pandemic, CNN report has said.
The news of the approval comes days after the President; Mr Donald Trump mounted pressure on the FDA to release the vaccine to save lives.
The 95% efficacy vaccine produced by an American multinational pharmaceutical corporation, Pfizer and a German biotechnology company, BioNTech, is expected to be distributed around the country in few days for emergency use with nursing home residents and health care workers listed to be the first to be administered with.
US FDA Boss speaks on the emergency authorization
A statement from the FDA Commissioner, Dr Stephen Hahn said the authorization of the vaccine marks a significant milestone in the country’s fight against the disease which has killed about 302,750 people in the country.
“The FDA’s authorization for emergency use of the first COVID-19 vaccine is a significant milestone in battling this devastating pandemic that has affected so many families in the United States and around the world”.
According to him, the emergency approval follows a thorough examination by industry experts for safety, effectiveness among others and attributed the achievement in the shortest possible time to scientific innovation and global collaboration.
“Today’s action follows an open and transparent review process that included input from independent scientific and public health experts and a thorough evaluation by the agency’s career scientists to ensure this vaccine met FDA’s rigorous, scientific standards for safety, effectiveness, and manufacturing quality needed to support emergency use authorization”.
“The tireless work to develop a new vaccine to prevent this novel, serious, and life-threatening disease in an expedited time frame after its emergence is a true testament to scientific innovation and public-private collaboration worldwide,” the statement added.
The vaccine, however requires a vote of recommendation from the US Centers for Disease Control and Prevention Immunization Advisory Committee and subsequent acceptance before vaccination could begin.
The COVID-19 Pandemic
The United State of America is one of the hardest hit of the coronavirus disease first recorded in China last year and spread through the world over early this year.
The country has recorded a total of 16,295,458 with more than 300,000 death as at December 12, this year.
Globally, the John Hopkins University report about 70 million confirmed cases in 190 countries with more than 1.5 million deaths.



Mahama leads NDC to mourn with Kufuor over wife’s passing
Former President John Dramani Mahama on Wednesday, October 4 led a delegation from the National Democratic Congress (NDC) to visit...


Former First Lady of Ghana Theresa Kufuor reported dead
The former First Lady of Ghana and wife of Ghana’s former President, John Agyekum Kufour, Mrs Theresa Kufuor has been...


The most powerful tool for change is the right to vote – Mahama
The 2024 flagbearer of the National Democratic Congress (NDC), John Dramani Mahama, is rallying Ghanaains who have grown disillusioned with...


PFJ was a mere state resource looting platform – Minority
The Minority has descended heavily on the Akufo-Addo-led government for launching a second phase of the flagship Planting for Food...


ECOWAS is desecrating Ghana
The Director of Legal Affairs of the National Democratic Congress (NDC), Mr Abraham Amaliba has said the Economy Community of...


Prof Gyampo elected President of UTAG, University of Ghana branch
A Senior Lecturer at the Political Science Department of the University of Ghana (Legon), Prof Ransford Gyampo have been elected...
